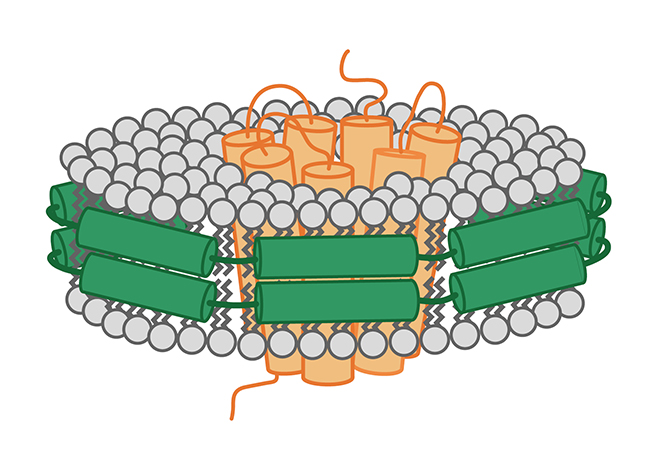
Nanodisc结合冷冻电镜应用时 ,Nanodisc提升了通过冷冻电镜对膜蛋白的解析率,同时表现了功能性磷脂所扮演的重要角色。
The last few years have seen a tremendous increase in high-resolution protein structures solved by cryo electron microscopy (cryo-EM). Novel electron detecting cameras and sophisticated analysis software have expanded the capacity of cryo-EM to smaller and asymmetric proteins (1). As a true competitor to X-ray crystallography, cryo-EM is particularly interesting for hard-to-crystallize targets such as membrane proteins.
在过去的几年里,使用冷冻电子显微镜(冷冻电镜)对蛋白结构高分辨率结构解析的应用有着很大地增长。新型的电子探测相机和复杂的分析软件使冷冻电镜的应用延伸到更小和不对称的蛋白结构解析(1)。作为X射线晶体法的真正强势的替代方法,冷冻电镜能把如膜蛋白等难以结晶的蛋白作为应用目标,并引起了各界广泛的兴趣。
The importance of sample preparation methods for high-resolution cryo-EM data cannot be underestimated. Two recent Nature publications have shown that nanodiscs are not only excellent tools for membrane protein stabilization, but that they can also improve resolution, in particular of the transmembrane region, and enable analysis of interacting phospholipids.
在应用的过程中,样品制备方法对得到高分辨率冷冻电镜数据的重要性是不可低估的。从最近的两篇发表到Nature的文章来看,Nanodisc不仅是膜蛋白稳定的优良工具,而且它也可以提高在电镜解析的分辨率,特别是膜蛋白的跨膜部分,同时能实现磷脂相互作用的分析。
Toxic: Near-atomic detail of a bacterial Tc toxin membrane insertion (2). Stefan Raunser's team at the Max-Planck Institute in Dortmund, Germany unveiled the mechanism used by bacterial Tc toxin as it enters the cell. Besides the high medical relevance of this project - Tc toxins include anthrax, plague, and scarlet-like fever toxins - the conformational changes these toxins undergo are simply fascinating. Secreted by bacteria as soluble proteins, toxins fold into channels that perforate the host membrane by a putative entropic spring mechanism. In previous attempts with detergent-solubilized protein, it was not possible to resolve the transmembrane region of the toxin. Now, using nanodisc-stabilized TcdA1 protein, researchers were able to achieve an overall resolution of 3.5 Angstrom, allowing them to describe this mechanically enforced membrane insertion mechanism for the first time.
Toxic: Near-atomic detail of a bacterial Tc toxin membrane insertion (2). 德国马克斯·普朗克研究所的Stefan Raunser团队阐述了细菌Tc毒素进入细胞的机制。除了这个项目的高度医学相关性价值外( Tc毒素包括炭疽,鼠疫,猩红热样毒素)这些毒素所经历的构象变化是有极大吸引力的。由细菌分泌的可溶性蛋白,毒素折叠成通道穿过宿主细胞膜。以前尝试使用去污剂溶解带跨膜区域蛋白进行分析,并不能很好地解析带跨膜区域的毒素。而现在使用Nanodisc稳定TcdA1蛋白,研究人员能够获得到3.5埃的解析度,这让他们有机会首先发现并描述了这种机械的强制膜插入的机制。
Hot & spicy: Functional lipids enable detection of heat and hot spices (3). Yifan Cheng's team at UCSF analyzed the tetrameric transient receptor potential vanilloid 1 (TRPV1) ion channel at 2.9 Angstrom resolution. TRPV1 reacts to many physical and chemical stimuli, including heat and capsaicin, an ingredient of chilli peppers. Nanodiscs were crucial to obtain a high resolution structure, as previous attempts with amphipol-stabilized complexes had only yielded a 3.8 A resolution.
But nanodiscs played another important role in this analysis: By providing a phospholipid bilayer, they enabled the discovery of lipids with a structural function in ligand binding. Similar to the results of the Dortmund group, the transmembrane regions were those with the highest resolution, stressing the value of nanodiscs for cryo-EM analysis.
Hot & spicy: Functional lipids enable detection of heat and hot spices (3). 加州大学旧金山分校的程亦凡团队分析了瞬态电压感受器阳离子通道1(TRPV1一种在疼痛和热知觉中起中心作用的蛋白质)在2.9埃分辨率离子通道结构。TRPV1对许多物理和化学刺激,包括热、辣椒素(一种辣椒的成分)都有反应。Nanodisc是此研究中获得高分辨率的结构十分重要的因素,而以前的尝试使用双极性稳定复合物只得到了3.8个埃的分辨率。
在这一研究中,Nanodisc还扮演了另一个非常重要的角色:通过提供一个磷脂双分子层,研究人员得以发现磷脂具有配基结合的结构功能。类似于Stefan Raunser团队的结果,跨膜区具有很高的分辨率,大大提升了Nanodisc在结合冷冻电镜分析膜蛋白应用中的价值。
参考文献:
1. Kühlbrandt, W. Cryo-EM enters a new era. eLIFE (2014) doi:10.7554/eLife.03678
2. Gatsogiannis, C. et al. Membrane insertion of a Tc toxin in near-atomic detail. Nature structural and molecular biology (2016),23,884-890. doi:10.1038/nsmb.3281
3. Gao, Y. et al. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature (2016) 534(7607):347-351. doi:10.1038/nature17964










 首页
首页 Facebook
Facebook WeChat
WeChat Twiitter
Twiitter
